Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis (FREEDOM Trial)
- The study enrolled 7868 women between the ages of 60 and 90 years who had a bone mineral density T score of less than −2.5 but not less than −4.0 at the lumbar spine or total hip
- Patients randomly assigned to receive either 60 mg of denosumab or placebo subcutaneously every 6 months for 36 months
- Primary end point: New vertebral fracture
- Secondary end points :Nonvertebral and hip fractures
Results: Denosumab given subcutaneously twice yearly for 36 months was associated with a reduction in the risk of vertebral, nonvertebral, and hip fractures in women with osteoporosis.
The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study
- All participants who completed the FREEDOM trial without discontinuing treatment or missing more than one dose of investigational product were eligible to enrol in the open-label, 7-year extension, in which all participants received denosumab
- 5928 (76%) women were eligible for enrolment in the extension, and of these, 4550 (77%) were enrolled (2343 long-term, 2207 crossover)
- Total 2626 women (1343 long-term; 1283 crossover) completed the extension
Outcome measures
- The primary outcome was safety monitoring, comprising assessments of adverse event incidence and serious adverse event incidence
- Secondary outcomes included new vertebral, hip, and non-vertebral fractures as well as bone mineral density (BMD) at the lumbar spine, total hip, femoral neck, and one-third radius
Efficacy Outcomes
- The yearly incidence of new vertebral fractures (ranging from 0·90% to 1·86%) and non-vertebral fractures (ranging from 0·84% to 2·55%) remained low during the extension, similar to rates observed in the denosumab group during the first three years of the FREEDOM study
-
In the long-term group, BMD increased from FREEDOM baseline by
- 21·7% at the lumbar spine
- 9·2% at total hip
- 9·0% at femoral neck
- 2·7% at the one third radius.
- In the crossover group, BMD increased from extension baseline by 16·5% at the lumbar spine, 7·4% at total hip, 7·1% at femoral neck, and 2·3% at one-third radius
Interpretation
Denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence compared with that observed during the original trial, and continued increases in BMD without plateau.
Comparative studies with Bisphosphonates
Comparison of the Effect of Denosumab and Alendronate on BMD and Biochemical Markers of Bone Turnover in Postmenopausal Women With Low Bone Mass: A Randomized, Blinded, Phase 3 Trial (DECIDE)
- The Determining Efficacy: Comparison of Initiating Denosumab versus Alendronate (DECIDE) trial
- This phase 3, multicenter, double blind study compared the efficacy and safety of denosumab with alendronate in postmenopausal women with low bone mass
-
1189 patients randomized (1:1) to
- Denosumab 60mg/6months
- Alendronate 70mg/wk
- Study duration: 12 months
-
End points
- Changes in BMD at total hip, femoral neck, trochanter, lumbar spine, and one-third radius at 6 and 12 months
- Bone turnover markers at months 1, 3, 6, 9, and 12
Outcomes
- Denosumab showed significantly larger gains in BMD at lumbar spine, total hip and femoral neck
- Denosumab showed significantly greater reduction in bone turnover markers compared with alendronate
- Adverse events and laboratory values were similar for denosumab and alendronate-treated subjects
Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis (FREEDOM Trial)
- The study enrolled 7868 women between the ages of 60 and 90 years who had a bone mineral density T score of less than −2.5 but not less than −4.0 at the lumbar spine or total hip
- Patients randomly assigned to receive either 60 mg of denosumab or placebo subcutaneously every 6 months for 36 months
- Primary end point: New vertebral fracture
- Secondary end points :Nonvertebral and hip fractures
Results: Denosumab given subcutaneously twice yearly for 36 months was associated with a reduction in the risk of vertebral, nonvertebral, and hip fractures in women with osteoporosis.
Effects of Denosumab on Bone Mineral Density and Bone Turnover in Postmenopausal Women Transitioning from Alendronate Therapy (STAND study)
- This was a multicenter, international, randomized, double-blind, double-dummy study in 504 postmenopausal women > or = 55 years of age with a BMD T-score of -2.0 or less and -4.0 or more who had been receiving alendronate therapy for at least 6 months
- Subjects received open-label branded alendronate 70 mg once weekly for 1 month and then were randomly assigned to either continued weekly alendronate therapy or subcutaneous denosumab 60 mg every 6 months and were followed for 12 months
- Significantly greater BMD gains with denosumab compared with alendronate also were achieved at 12 months at the lumbar spine, femoral neck, and 1/3 radius (all p < .0125)
- Median serum CTX levels remained near baseline in the alendronate group and were significantly decreased versus alendronate (p < .0001) at all time points with denosumab
- Adverse events and serious adverse events were balanced between groups
DAPS (Denosumab Adherence Preference Satisfaction) study
Osteoporosis patients who are non-compliant or non-persistent with therapy may have suboptimal clinical outcomes. This 2-year, randomized, open-label, crossover study compared treatment adherence between subcutaneous denosumab, 60 mg every 6 months, and oral alendronate, 70 mg once weekly among 250 PMO patients.
Results
-
Denosumab treated patients as compared to alendronate treated patients had
- 80% lower risk of non-adherence
- 80% RRR (Relative risk reduction) of non-compliance
- 91% RRR of non-persistence
- 92.4% patients preferred the injections over the oral therapy
Denosumab or Zoledronic Acid in Postmenopausal Women with Osteoporosis Previously Treated with Oral Bisphosphonates
- The objective of the study was to compare the effect of transitioning from oral bisphosphonates to denosumab or ZOL on bone mineral density (BMD) and bone turnover
- A total of 643 postmenopausalwomenwith osteoporosis previously treated with oral bisphosphonates participated in the study
- Subjects were randomized 1:1 to sc Denosumab 60mg every 6 months plus iv placebo once or zoledronic acid 5 mg iv once plus sc placebo every 6 months for 12 months
Results
- BMD change from baseline at month 12 was significantly greater with denosumab compared with ZOL at Lumbar spine, total hip, femoral neck, one-third radius
- The median decrease from baseline was greater with denosumab than ZOL for serum C-telopeptide of type 1 collagen at all time points after day 10 and for serum procollagen type 1 N-terminal propeptide at month 1 and at all time points after month 3 (all P<0.05)
Denosumab and Teriparatide
Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial
- 100 Postmenopausal women with osteoporosis enrolled
-
Patients were assigned in a 1:1:1 ratio
- 20 μg teriparatide daily
- 60 mg denosumab every 6 months
- Both of the above
- BMD was measured at 0, 3, 6, and 12 months
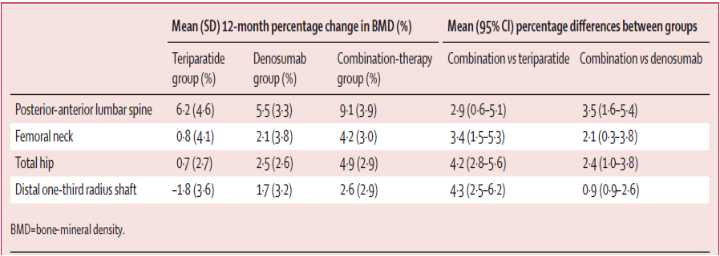
Changes in bone mineral density and differences between groups at 12months
Two Years of Denosumab and Teriparatide Administration in Postmenopausal Women With Osteoporosis (The DATA Extension Study): A Randomized Controlled Trial
- Preplanned continuation of the Denosumab and Teriparatide Administration (DATA)
- 94 postmenopausal women with osteoporosis
-
Outcome Measures
- Lumbar spine, femoral neck, total hip, and distal radius BMD
- Serum markers of bone turnover
Results
- Two years of concomitant Teriparatide and Denosumab therapy increases BMD
- More than therapy with either medication alone
- More than that has been reported with any of currently existent therapy

