- A Multi Centric, Randomized, Assessor Blind, Parallel, Phase III Study to Evaluate Clinical Efficacy, Safety and Pharmacokinetics of Intas Denosumab (60 mg Single Use Vial) in Comparison to The Reference Listed Drug Prolia® (Denosumab, 60 mg Single Use Vial) in Women With Postmenopausal Osteoporosis
- 1 month screening period and 12 months of treatment phase
- Eligible patients received Denosumab of Intas Pharmaceuticals Limited, India or the innovator product Prolia after randomization
- Treatment: Denosumab 60 mg 6 monthly + Calcium 1000mg/day + Vitamin D 400IU/day
- Efficacy and safety evaluation was done at the end of 12 months
- Pharmacokinetic evaluation for both the drugs was done till the end of 6 months (Day 168)
Efficacy outcomes
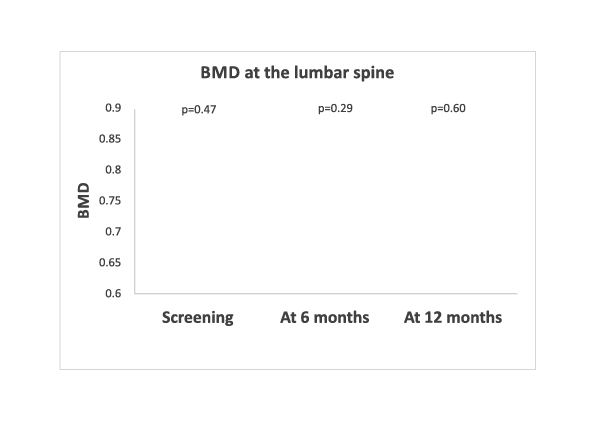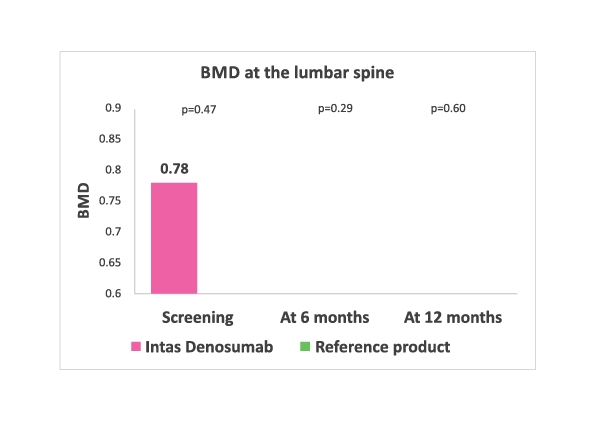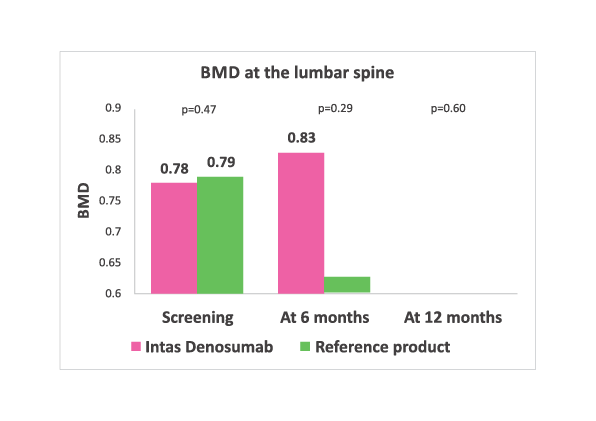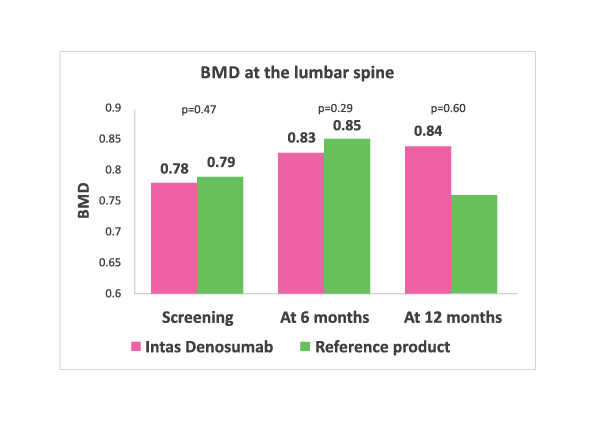
Lumbar spine BMD values at baseline, 6 months and 12 months in both the treatment arms. No significant difference in two groups at any time point.
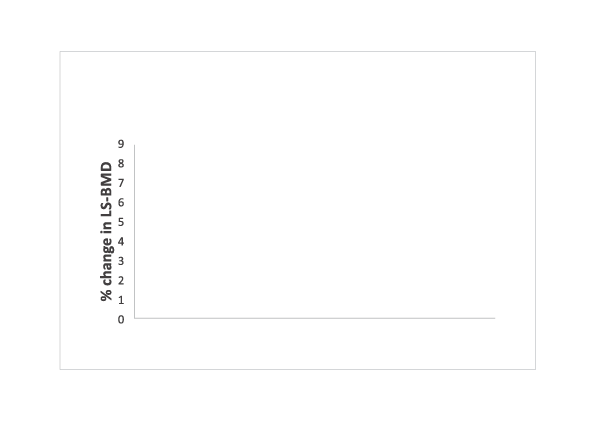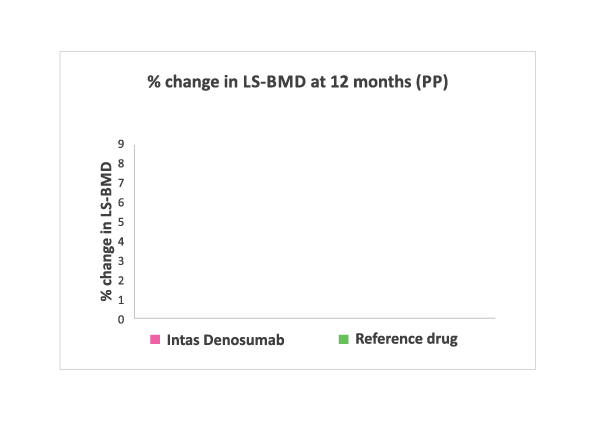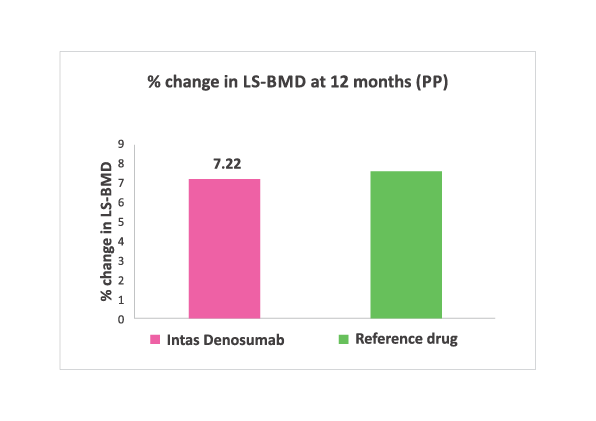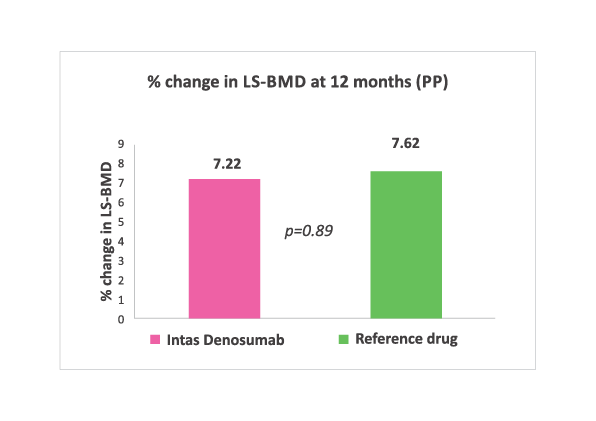
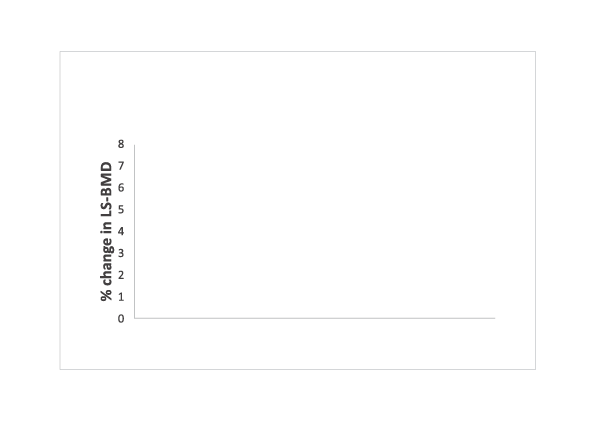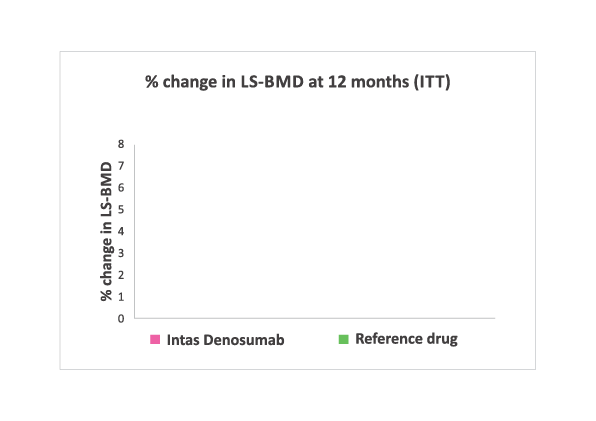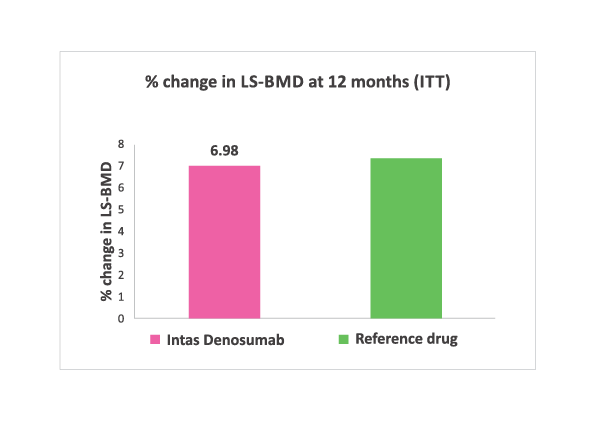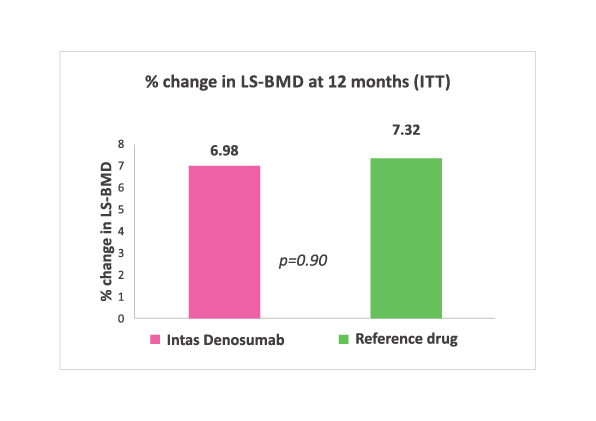
% change in LS-BMD at 12 months. No significant difference (Both Intention to treat and per protocol analysis) noted between two groups.
Pharmacokinetic evaluation
- There was no significant difference noted in pharmacokinetic parameters like AUC0-t, AUC0-∞ , Cmax and Tmax of test drug and reference drug
Safety and Immunogenicity
- Test and the Reference products were found well tolerated. No patient was positive for antidrug antibodies
Conclusion
- Intas Denosumab is efficacious and comparable to the reference drug, Prolia of Amgen Inc., in terms of efficacy, safety, and PK profiles

